By Peter A. McCullough, MD, MPH
As an epidemiologist I am dedicated to determining the scientific validity of published studies. Schmeling's analysis showing that the batch or many of Pfizer's mRNA vaccines appeared to have highly unbalanced side effect profiles was outwardly consistent with the VAERS data presented by Senator Ron Johnson at the CDC. This has been the main explanation for why some are well and others are injured or dead after being vaccinated against COVID-19. But was there any confirmation of what Schmeling et al found?
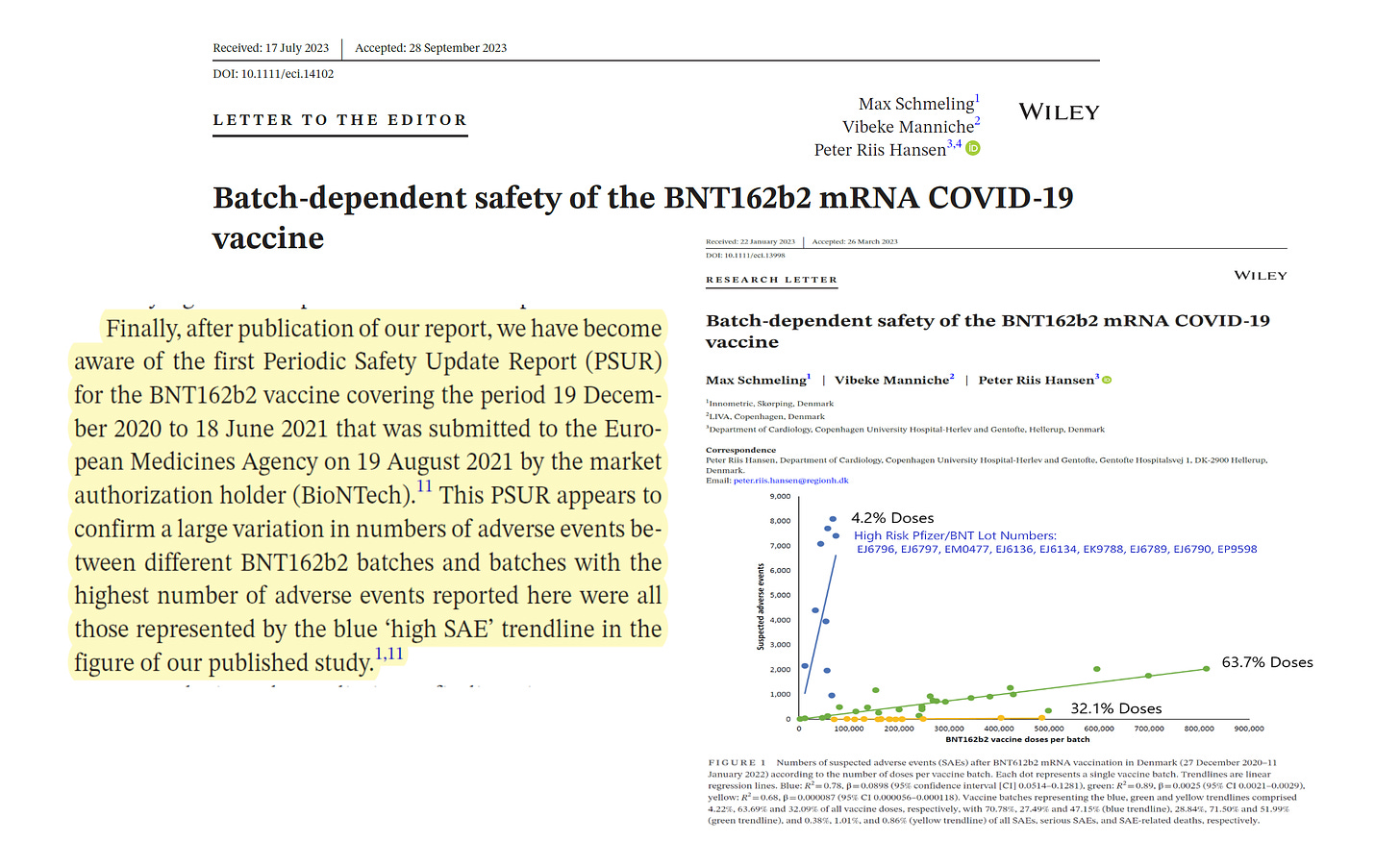
In a response to a letter to the editor, Schmeling et al disclose Pfizer's Periodic Safety Update as a source of internal validity.
“Finally, following the publication of our report, we have become aware of the first Periodic Safety Update Report (PSUR) for the BNT162b2 vaccine covering the period from December 19, 2020 to June 18, 2021 that was submitted to the European Medicines Agency on 19 August 2021. by the holder of the market authorization (BioNTech).11 This PSUR appears to confirm a large variation in the number of adverse events between different batches of BNT162b2 and the batches with the highest number of adverse events reported here were all those represented by the blue trendline “high SAE” in the figure from our published study”.
So it seems that the “hot batch” theory is confirmed. This explains why approximately 32% have virtually zero side effects and have little cause for concern. We just wish everyone could be in this group. Everyone else has some reason to guard personal health in the event of vaccine-induced medical problems.
Please subscribe brave speech as a paying member or founder so we can continue to bring you the truth.
Peter A. McCullough, MD, MPH
President of the McCullough Foundation